Information from CDC
Biological Toxins. Biological toxins (also referred to as biotoxins) are nonliving toxic proteins that are naturally produced by many different types of living organisms. Biotoxins are:
- Thousands of times more toxic by mass than chemical warfare agents.
- Considered to pose the same level of risk as the microorganisms that
produce them.
- Not themselves infectious or contagious after exposure. However, a biotoxin-producing organism may be infectious or contagious after exposure.
Bacterial Toxins: Friends or Foes?
Clare K. Schmitt, Karen C. Meysick, and Alison D. O'Brien
Uniformed Services University of the Health Sciences, Bethesda, Maryland,
USA
| Many emerging and reemerging
bacterial pathogens synthesize toxins that serve as primary virulence
factors. We highlight seven bacterial toxins produced by
well-established or newly emergent pathogenic microbes. These toxins,
which affect eukaryotic cells by a variety of means, include
Staphylococcus aureus
|
Since diphtheria toxin was isolated by Roux and Yersin in 1888, microbial toxins have been recognized as the primary virulence factor(s) for a variety of pathogenic bacteria. Bacterial toxins have been defined as "soluble substances that alter the normal metabolism of host cells with deleterious effects on the host". Indeed, the major symptoms associated with disease caused by Corynebacterium diphtheriae (diphtheria), Bordetella pertussis (whooping cough), Vibrio cholerae (cholera), Bacillus anthracis (anthrax), Clostridium botulinum (botulism), Clostridium tetani (tetanus), and enterohemorrhagic Escherichia coli (bloody diarrhea and hemolytic uremic syndrome) are all related to the activities of the toxins produced by these organisms. With the recognition of the central role of toxin in these and other diseases has come the application of inactive toxins (toxoids) as vaccines. Such toxoid vaccines have had an important positive impact on public health.
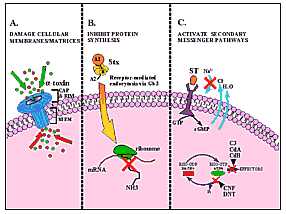
In this review, we provide a summary overview (Table)
of a variety of bacterial toxins categorized according to mode of action:
damaging cell membranes, inhibiting protein synthesis, activating second
messenger pathways, inhibiting the release of neurotransmitters, or
activating the host immune response. We also describe in detail seven
toxins: Staphylococcus aureus
![]() -toxin,
Shiga toxin (Stx), cytotoxic necrotizing factor type 1 (CNF1), E. coli
heat-stable toxin (ST), botulinum and tetanus neurotoxins, and toxic-shock
syndrome toxin (TSST) produced by S. aureus. We emphasize these
toxins because they are produced by emerging (Stx of enterohemorrhagic E.
coli) or reemerging (
-toxin,
Shiga toxin (Stx), cytotoxic necrotizing factor type 1 (CNF1), E. coli
heat-stable toxin (ST), botulinum and tetanus neurotoxins, and toxic-shock
syndrome toxin (TSST) produced by S. aureus. We emphasize these
toxins because they are produced by emerging (Stx of enterohemorrhagic E.
coli) or reemerging (![]() -toxin
of multidrug-resistant S. aureus) pathogens or illustrate different
structures or modes of action (ST, CNF1, neurotoxins, and TSST).
-toxin
of multidrug-resistant S. aureus) pathogens or illustrate different
structures or modes of action (ST, CNF1, neurotoxins, and TSST).
| Table. Characteristics of bacterial toxinsa | ||||
| Organism/toxin | Mode of action | Target | Disease | Toxin implicated in diseaseb |
| Damage membranes | ||||
| Aeromonas hydrophila/aerolysin |
Pore-former | Glycophorin | Diarrhea | (yes) |
| Clostridium perfringens/ |
Pore-former | Cholesterol | Gas gangrenec | ? |
| perfringolysin O | ||||
| Escherichia coli/ hemolysind |
Pore-former | Plasma membrane | UTIs | (yes) |
| Listeria monocytogenes/ | Pore-former | Cholesterol | Foodborne systemic | (yes) |
| listeriolysin O | illness meningitis | |||
| Staphyloccocus aureus/ |
Pore-former | Plasma membrane | Abcessesc | (yes) |
| Streptococcus pneumoniae/ |
Pore-former | Cholesterol | Pneumoniac | (yes) |
| pneumolysin | ||||
| Streptococcus pyogenes/ | Pore-former | Cholesterol | Strep throat Sfc | ? |
| streptolysin O | ||||
| Inhibit protein synthesis | ||||
| Corynebacterium diphtheriae/ |
ADP- ribosyltransferase |
Elongation factor 2 | Diphtheria | yes |
| diphtheria toxin | ||||
| E. coli/Shigella dysenteriae/ |
N-glycosidase | 28S rRNA | HC and HUS | yes |
| Shiga toxins | ||||
| Pseudomonas aeruginosa/ |
ADP- ribosyltransferase |
Elongation factor 2 | Pneumoniac | (yes) |
| exotoxin A | ||||
| Activate second messenger pathways | ||||
| E.coli | ||||
| CNF | Deamidase | Rho G-proteins | UTIs | ? |
| LT | ADP- ribosyltransferase |
G-proteins | Diarrhea | yes |
| STd | Stimulates guanylate cyclase |
guanylate cyclase receptor |
Diarrhea | yes |
| CLDTd | G2 block | Unknown | Diarrhea | (yes) |
| EAST | ST-like? | Unknown | Diarrhea | ? |
| Bacillus anthracis/ edema factor |
Adenylate cyclase | ATP | Anthrax | yes |
| Bordetella pertussis/ | ||||
| dermonecrotic toxin | Deamidase | Rho G-proteins | Rhinitis | (yes) |
| pertussis toxin | ADP- ribosyltransferase |
G-protein(s) | Pertussis | yes |
| Clostridium botulinum/ C2 toxin |
ADP- ribosyltransferase |
Monomeric G-actin | Botulism | ? |
| C. botulinum/C3 toxin | ADP- ribosyltransferase |
Rho G-protein | Botulism | ? |
| Clostridium difficile/ | ||||
| toxin A | Glucosyltransferase | Rho G-protein(s) | Diarrhea/PC | (yes) |
| toxin B | Glucosyltransferase | Rho G-protein(s) | Diarrhea/PC | ? |
| Vibrio cholerae/cholera toxin |
ADP- ribosyltransferase |
G-protein(s) | Cholera | yes |
| Activate immune response | ||||
| S. aureus/ | ||||
| enterotoxins | Superantigen | TCR and MHC II | Food poisoningc | yes |
| exfoliative toxins | Superantigen (and serine protease?) |
TCR and MHC II | SSSc | yes |
| toxic-shock toxin | Superantigen | TCR and MHC II | TSSc | yes |
| S. pyogenes/pyrogenic exotoxins |
Superantigens | TCR and MHC II | SF/TSSc | yes |
| Protease | ||||
| B. anthracis/lethal factor | Metalloprotease | MAPKK1/MAPKK2 | Anthrax | yes |
| C. botulinum/neurotoxins A-G | Zinc-metalloprotease | VAMP/synaptobrevin SNAP-25 syntaxin |
Botulism | yes |
| Clostridium tetani/ tetanus toxin |
Zinc-metalloprotease | VAMP/synaptobrevin | Tetanus | yes |
| aAbbreviations:
CNF, cytotoxic necrotizing factor; LT, heat-labile toxin; ST,
heat-stable toxin; CLDT, cytolethal distending toxin; EAST,
enteroaggregative E. coli heat-stable toxin; TCR, T-cell
receptor; MHC II, major histocompatibility complex class II; MAPKK,
mitogen-activated protein kinase kinase; VAMP, vesicle-associated
membrane protein; SNAP-25, synaptosomal-associated protein; UTI, urinary
tract infection; HC, hemorrhagic colitis; HUS, hemolytic uremic
syndrome; PC, antibiotic-associated pseudomembranous colitis; SSS,
scalded skin syndrome; SF, scarlet fever; TSS, toxic-shock syndrome. bYes, strong causal relationship between toxin and disease; (yes), role in pathogenesis has been shown in animal model or appropriate cell culture; ?, unknown. cOther diseases are also associated with the organism. dToxin is also produced by other genera of bacteria |
||||
When It Rains, It Pores
Many bacterial exotoxins have the capacity to damage the extracellular
matrix or the plasma membrane of eukaryotic cells. The damage not only may
result in the direct lysis of cells but also can facilitate bacterial spread
through tissues. Toxins that mediate this cellular damage do so by either
enzymatic hydrolysis or pore formation. Bacterial hyaluronidases,
collagenases, and phospholipases have the capacity to degrade cellular
membranes or matrices. Specific examples of these types of toxins include
the ![]() -toxin
of Clostridium perfringens, which has phospholipase C activity;
Streptococcus pyogenes streptokinase, which can hydrolyze
plasminogen to plasmin and dissolve clots; and the clostridial collagenases.
Pore-forming toxins, as the name suggests, disrupt the selective influx and
efflux of ions across the plasma membrane by inserting a transmembrane pore.
This group of toxins includes the RTX (repeats in toxin) toxins from
gram-negative bacteria, streptolysin O produced by S. pyogenes, and
the S. aureus
-toxin
of Clostridium perfringens, which has phospholipase C activity;
Streptococcus pyogenes streptokinase, which can hydrolyze
plasminogen to plasmin and dissolve clots; and the clostridial collagenases.
Pore-forming toxins, as the name suggests, disrupt the selective influx and
efflux of ions across the plasma membrane by inserting a transmembrane pore.
This group of toxins includes the RTX (repeats in toxin) toxins from
gram-negative bacteria, streptolysin O produced by S. pyogenes, and
the S. aureus
![]() -toxin
(described below).
-toxin
(described below).
S. aureus
![]() -toxin
can be considered the prototype of oligomerizing pore-forming cytotoxins.
The
-toxin
can be considered the prototype of oligomerizing pore-forming cytotoxins.
The ![]() -toxin
gene resides as a single copy on the chromosome of most pathogenic S.
aureus strains, and its expression is environmentally regulated at the
transcriptional level by the staphylococcal accessory gene regulator
(agr) locus. The
-toxin
gene resides as a single copy on the chromosome of most pathogenic S.
aureus strains, and its expression is environmentally regulated at the
transcriptional level by the staphylococcal accessory gene regulator
(agr) locus. The
![]() -toxin
is synthesized as a 319 amino acid precursor molecule that contains an
N-terminal signal sequence of 26 amino acids. The secreted mature toxin, or
protomer, is a hydrophilic molecule that lacks cysteine residues and has a
molecular mass of approximately 33 kDa. Recently, the crystallographic
structure of the fully assembled
-toxin
is synthesized as a 319 amino acid precursor molecule that contains an
N-terminal signal sequence of 26 amino acids. The secreted mature toxin, or
protomer, is a hydrophilic molecule that lacks cysteine residues and has a
molecular mass of approximately 33 kDa. Recently, the crystallographic
structure of the fully assembled
![]() -toxin
pore was solved. On the plasma membrane, seven toxin protomers assemble to
form a 232 kDa mushroom-shaped heptamer comprising three distinct domains (Figure
1A). The cap and rim domains of the
-toxin
pore was solved. On the plasma membrane, seven toxin protomers assemble to
form a 232 kDa mushroom-shaped heptamer comprising three distinct domains (Figure
1A). The cap and rim domains of the
![]() -toxin
heptamer are situated at the surface of the plasma membrane, while the stem
domain serves as the transmembrane channel.
-toxin
heptamer are situated at the surface of the plasma membrane, while the stem
domain serves as the transmembrane channel.
Alpha-toxin is cytolytic to a variety of cell types, including human
monocytes, lymphocytes, erythrocytes, platelets, and endothelial cells. For
![]() -toxin
to damage cellular membranes, three sequential events are required. Toxin
protomers must first bind to target membranes by either unidentified
high-affinity receptors or through nonspecific absorption to substances such
as phosphotidylcholine or cholesterol on the lipid bilayer. Second,
membrane-bound protomers must oligomerize into a nonlytic prepore heptamer
complex. Third, the heptamer must undergo a series of conformational changes
that create the stem domain of the toxin, which is then inserted into the
membrane. The
-toxin
to damage cellular membranes, three sequential events are required. Toxin
protomers must first bind to target membranes by either unidentified
high-affinity receptors or through nonspecific absorption to substances such
as phosphotidylcholine or cholesterol on the lipid bilayer. Second,
membrane-bound protomers must oligomerize into a nonlytic prepore heptamer
complex. Third, the heptamer must undergo a series of conformational changes
that create the stem domain of the toxin, which is then inserted into the
membrane. The
![]() -toxin
pore allows the influx and efflux of small molecules and ions that
eventually lead to the swelling and death of nucleated cells and the osmotic
lysis of erythrocytes. Pore formation has also been shown to trigger
secondary events that could promote development of pathologic sequelae.
These events include endonuclease activation, increased platelet exocytosis,
release of cytokines and inflammatory mediators, and production of
eicosanoids. Several animal models have demonstrated that
-toxin
pore allows the influx and efflux of small molecules and ions that
eventually lead to the swelling and death of nucleated cells and the osmotic
lysis of erythrocytes. Pore formation has also been shown to trigger
secondary events that could promote development of pathologic sequelae.
These events include endonuclease activation, increased platelet exocytosis,
release of cytokines and inflammatory mediators, and production of
eicosanoids. Several animal models have demonstrated that
![]() -toxin
is required for S. aureus virulence in these systems; however, the
precise role of
-toxin
is required for S. aureus virulence in these systems; however, the
precise role of
![]() -toxin
in staphylococcal diseases in humans remains unclear.
-toxin
in staphylococcal diseases in humans remains unclear.
Stop, in the Name of Toxin
A second class of toxins intoxicates target cells by inhibiting protein synthesis. Substrates for toxins in this group are elongation factors and ribosomal RNA. Diphtheria toxin and Pseudomonas exotoxin A act by ADP-ribosylating elongation factor 2 (EF2). The modified EF2 is no longer able to function in protein synthesis. Stxs, also called verotoxins, are produced by Shigella dysenteriae serotype 1 and the emerging pathogens designated Stx-producing E. coli (STEC). Stxs inactivate ribosomal RNA (by a mechanism described below) so that the affected ribosome can no longer interact with elongation